Partner with Javara for the power of predictability
Diverse patient populations
We provide access to broader and more diverse patient populations at the community level for representative results.
Higher enrollment and retention
Patients are connected to clinical trials through their trusted physician and provided personalized support throughout their journey, resulting in higher enrollment and retention.
Consistent quality and delivery
Experienced Clinical Trial Navigators are integrated at the point of care to partner with investigators and manage trial conduct for reliable data and outcomes.
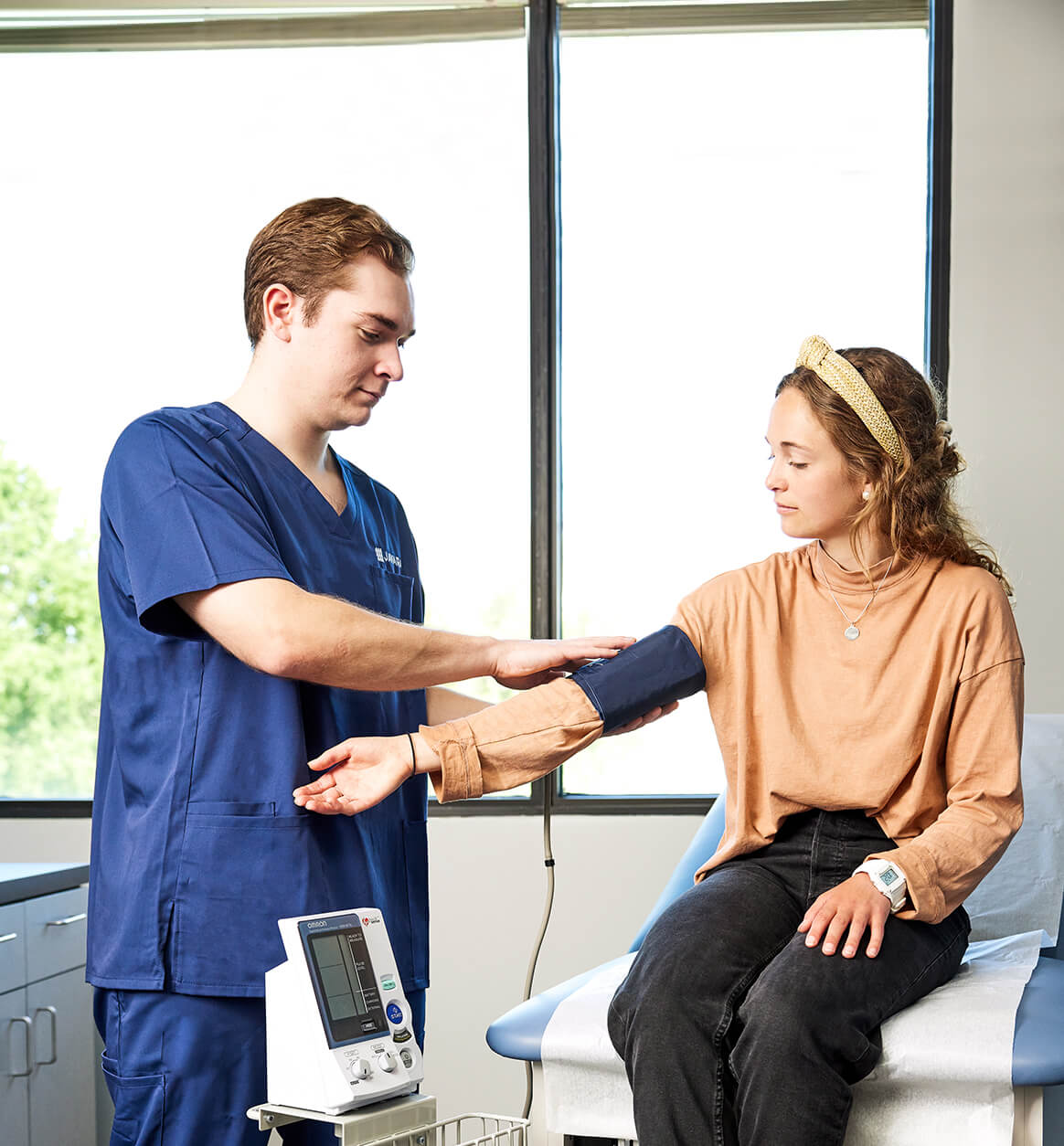
Reaching over 5 million patients and 5,800 practitioners with access to clinical research at the point of care
Delivering Efficiencies in Clinical and Decentralized Settings
Value-Driven Feasibility
- Behind-the-firewall EHR access for rapid protocol matching with identified patients
- Ability to map out the best locations for research access

Accelerated Start-up
- One contract template and one per-patient budget across the network
- Centralized resources for Regulatory, Contracts, Training, and Activation

Quality Delivery
- Standardized SOPs across the network
- Tailored quality management plans
- Proprietary training programs and workflows
Data Management
- Data entry and query resolution standards
- Internal data review and study monitoring
- Remote monitoring for CRAs

Optimized Technologies
- eRegulatory
- eSource
- Telehealth
Technology
- eConsent capabilities
- Decentralized and virtual capabilities
- eRegulatory management via Florence
- Utilization of eSource and CTMS platform via Clinical Conductor
- Study start-up and performance analytics via Devana Solutions
- Firewalls in place to ensure safe and secure internet connectivity
- Part 11 Compliant
- Multi-factor authentication and other measures to ensure information security
- Quality output and uniformity through Standard Operating Procedures (SOPs)
- Secure servers (including 24/7 surveillance and bio-metric access)
- Secure Socket Layer (SSL) data encryption
- Wireless internet access
- Stipend payments through ClinCard
Facility Specs & Equipment
Routine calibration is conducted on all research equipment.
- Ample supply storage
- Ambient and refrigerated centrifuges
- CLIA waived lab facilities
- Dedicated monitoring space
- Comprehensive imaging capabilities
- Electrocardiogram
- Freezers and refrigerators
- Secure document storage
- Local lab capabilities
- Emergency equipment (AED)
- Secured climate-controlled investigational product storage
- Continuous temperature monitoring of drug storage area and refrigerated units using SmartSense by Digi
How Javara supports investigators
Javara provides 360° training and support systems for experienced and emerging investigators through a mix of remote and onsite resources including oversight and mentoring.
40% of physicians leave traditional clinical research after a single trial
96% of Javara Investigators have remained in research after their first trial
Partner With Us
With the ever-increasing complexities in clinical research, the Javara model provides innovative solutions for sponsors and CROs. Delivering Clinical Research As A Care Option (CRAACO) through strategic partnerships, Javara’s turn-key integration of site staff and infrastructure is transforming the future of clinical research. Hear from Javara Board Member Jeff Kasher, PhD as he shares more about Javara through the lens of an executive with extensive experience in the pharmaceutical industry.
A video is being shown







