Case Study November 16, 2020
Investigative Site Placement Practices to Support Operation Warp Speed
Comparing late-stage COVID-19 vaccine trials to historical practices.
Introduction
Of the more than 1,600 active clinical trials of treatments and vaccines for SARS-CoV-2 (COVID-19), four vaccine trials are in late stage (Phase III) activity. These promising candidates have received funding through the Operation Warp Speed (OWS) program from the US federal government. The primary aim of OWS is to rapidly develop and distribute a sufficient amount of safe and effective vaccine doses to immunize the U.S. population against COVID-19.
A look at the placement and conduct of these late-stage clinical trials offers insight into site engagement practices necessitated by global public health urgency. What do these site engagement practices tell us about sponsor confidence in meeting extremely aggressive enrollment timelines and demanding trial execution requirements? Do these practices signal a shift in where sponsors can be expected to conduct clinical trials in the future?
This article summarizes the results of a study that we have performed to answer these questions. Our goal is to assess COVID-19 site placement practices and to compare them with historical practices.
Classifying Phase III COVID-19 vaccine trials site segments
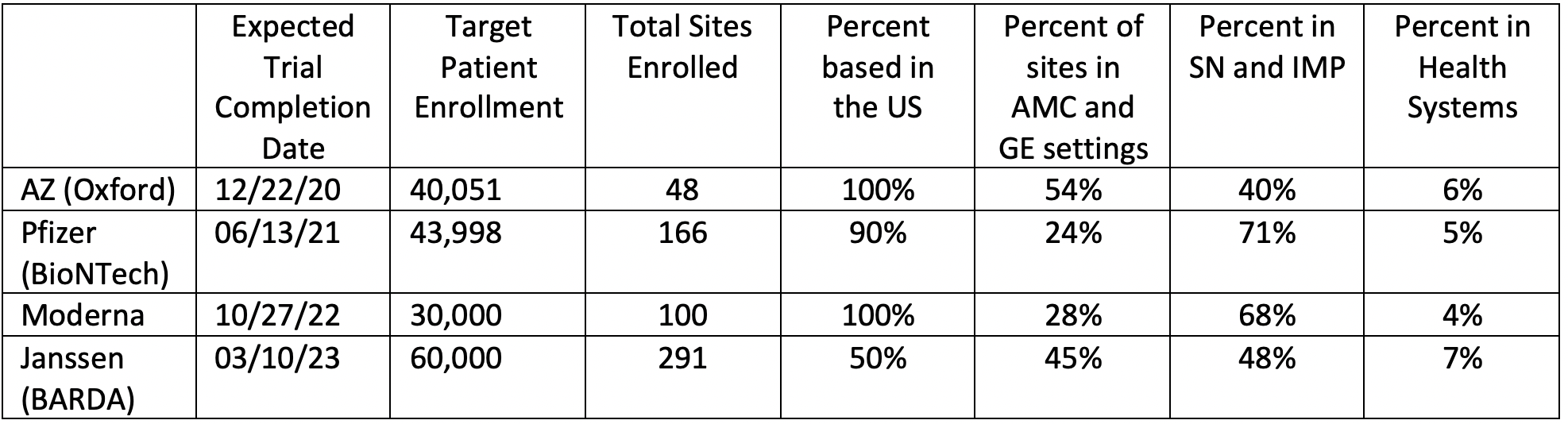
For this assessment, we have included all locations where Phase III COVID-19 vaccine studies, under Operation Warp Speed, are actively enrolling patients as of October 8th. Sponsors who were engaged in studies fitting these criteria are Moderna, Inc., Janssen Pharmaceuticals (in partnership with the Biomedical Advanced Research and Development Authority or BARDA), Pfizer, Inc. (with BioNTech), and AstraZeneca (with Oxford University).
We relied on data from ClinicalTrials.gov to identify the investigative sites participating in each of these four active Phase III COVID-19 vaccine trials. Although investigative sites in these trials continue to change, this study examines all sites that were actively enrolling as of October 8th. Data from ClinicalTrials.gov was supplemented by press releases, news articles, and other publicly available information to segment investigative sites into five categories based on the following definitions:
- Site Networks (SN): defined as a collective of independent sites that derive the majority of their income from clinical research grants.This includes dedicated independent sites that are permanently part of, or affiliated with, a network. Sites within this category are generally for-profit organizations;
- Academic Medical Center (AMC): defined as any site which has a major affiliation with a teaching institution. This includes any site that is a member of the Association of American Medical Colleges as a Medical School Member or a Hospital/Health System Member. Sites within this category are generally not-for-profit organizations;
- Health System: defined as any entity that is, or is affiliated with, a system that includes a hospital. Sites within this category are both for-profit and not-for-profit entities. Some for-profit health systems have established not-for-profit entities within themselves for the purpose of carrying out clinical research, a factor that makes this segment difficult to classify;
- Independent Medical Practice (IMP): defined as any community-based, independent clinical care provider that is not part of a larger health system. Sites within this category are generally for-profit organizations;
- Government Entity (GE): defined as any site that is funded and administered by the federal government. The two major types of government entities include Veterans Affairs organizations and Indian Health Service affiliates. Sites within this category are not-for-profit organizations.
Sites supporting AstraZeneca and Oxford University’s COVID-19 clinical trial were not named in ClinicalTrials.gov; in the US, only the zip code and city name were provided.To identify and classify these sites, we used not only press releases and local news articles, but also cross-referenced the AZ site locations with sites participating in the other three sponsors’ studies.
Late-stage COVID-19 trial placement
Overall, we found that approximately half (53%) of Operation Warp Speed (OWS) clinical trials are placed within site networks; one-third (34%) of clinical trials are placed within academic medical centers; 5% within health systems; and the remaining 8% are roughly evenly placed within government entities and independent medical practices (see Table 1 below).
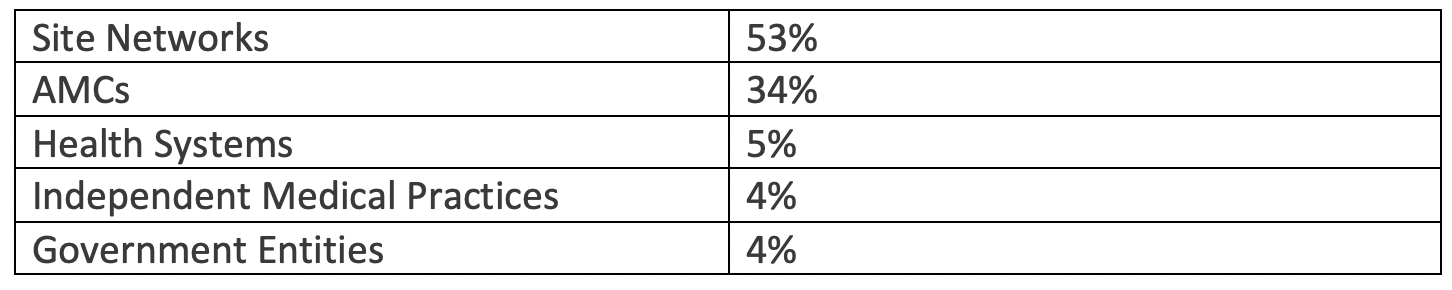
With the exception of Janssen’s late stage COVID clinical trials, site networks and academic medical centers operating in the US were the dominant two settings. SNs and AMCs combined to account for almost 90% of all clinical trial settings for AZ, Pfizer and Moderna programs; these two segments together account for 75% of sites conducting the Janssen clinical trial. For-profit site networks were the largest category in every clinical trial except for those sponsored by AstraZeneca, where AMCs comprised the majority.
Site networks made up at least two-thirds of both Moderna and Pfizer’s study sites; whereas they only accounted for about 40% of sites in Janssen and AstraZeneca’s clinical trials. The similar site placement patterns observed in Moderna and Pfizer’s clinical trials may in part be due to their use of the same contract research organization (PPD).
Academic Medical Centers account for over half of AstraZeneca’s sites (52%), making them the only sponsors to have engaged more AMCs than SNs.
Janssen has almost five times as many government entities as the next closest sponsor with 10% of all clinical trials being placed within federally-funded institutions. This is in part due to Janssen and BARDA’s higher relative utilization of Veterans Affairs offices.
Table 2 below shows that Janssen’s COVID-19 clinical trial is the most evenly dispersed across for-profit and not-for-profit organizations. Moderna and Pfizer are the most heavily weighted toward for-profit investigative site settings (68-70%) vs. not-for-profit settings (24%-28%).
Comparing COVID-19 trial placement to past placement practice
The most striking observation in this study is the remarkable consistency of placement practices over time including those testing new treatments and vaccines for COVID-19. In 2013, a study from the Tufts Center for the Study of Drug Development (CSDD) noted that “53% of all global FDA-regulated clinical trials are now conducted by independent, community-based principal investigators”.1 This 53% share of the total site landscape is identical to the share captured by site networks in COVID-19 vaccine trials.
Academic medical centers have held on to a more modest share of industry-funded clinical trials. In a 2007 report, Tufts CSDD noted that “today, only one-third of all industry-funded clinical trials are placed within academic clinical trials offices”.2 Our study found a similar percentage —34% — of late stage COVID-19 vaccine clinical trials are being conducted within academic settings.
Academic medical centers have not been alone in their inability to increase market share of industry-funded clinical trials. Government entities — such as Veterans Affairs offices — have captured an even smaller share of this market. This is due in part to the protracted contracting, negotiations, and bureaucracy that historically contribute to slower study start-up times there when compared to that of site networks and dedicated investigative sites.3 Academic medical centers have leveraged their experience, prestige, infrastructure, and patient volume to maintain their market share. The VA setting, on the other hand, has been a consistently less attractive place for industry-funded trials.4 During the pandemic, with increased interest in public-private collaboration, the VA setting for industry-funded clinical trials will likely receive greater visibility.
Comparing sponsors of late stage COVID trials, those companies most closely collaborating with government and non-profit partners (e.g., AstraZeneca and Janssen) have placed their clinical trials in a higher relative percentage of academic medical centers. In the case of AstraZeneca, this decision is consistent with the company’s historical site placement practice: In 2007 AstraZeneca’s R&D director Ellis Wilson remarked that, “by mitigating bottlenecks on the critical path, academic sites are becoming more attractive and may even start to account for a growing proportion of trial placements. The pendulum is swinging back in their direction. And pharmaceutical companies want to be their partner of choice”.2
Discussion
In the past, sponsors have relied on independent, for-profit investigative sites when speed and efficiency are paramount. In today’s COVID-19 vaccine landscape, the same holds true. Moderna and Pfizer’s studies, with their heavy reliance on for-profit sites, are moving the fastest in the race to prove the safety and efficacy of their vaccines.
Given the consistency observed in investigative site placement practices, we can assume that there will be a similar consistency in sponsor experience with participant diversity and inclusion for their COVID-19 trials. We can expect to see the same levels of proportional representation by participant demographic subgroup: White and Asian participants will be overrepresented, and participants from minority communities — most notably those of Black and African descent — will be underrepresented. This is particularly concerning given the higher observed rate and severity of COVID-19 cases among minority communities. Indeed, a lack of diversity has already been documented in both the NIAID Adaptive Covid-19 Treatment Trial (ACTT-1) and the Gilead-funded remdesivir study.5
It is interesting to note the very low percentage of investigative sites from health systems and independent medical centers participating in these late stage COVID-19 clinical trials. Although these segments offer access to a large volume of patients within clinical care settings, combined they account for less than 10% of total sites. Similar to pre-pandemic conditions, these entities have been less attractive settings when compared to site networks and academic medical centers that offer larger numbers and more dedicated personnel; higher levels of experience and competency; and substantially greater research infrastructure.6 Hospitals and independent medical centers were also the slowest to respond during the early stages of lockdowns throughout the US, and these sites were among the most likely to suspend their clinical trials at the outset of the pandemic.7
Community hospitals often find themselves being passed over during site selection in favor of academic medical centers which boast experienced investigators and specialized researchers.8 This is another reason why new investigators and health systems are not participating in COVID-19 clinical trials: sponsors place a high value on prior experience rather than total potential for accessing and attracting a large and diverse patient population. This fact makes it difficult for new investigators and sites to get their foot in the door for new clinical trials, and nowhere is this trend reflected more than in this research. Sponsors and CROs are choosing to place their study sites within entities that have proven track records, and the result is that site networks and AMCs account for over 85% of late stage COVID-19 vaccine trial sites.
Some independent medical practices and health systems are turning to partnership as a way to win and participate in more trials. The integrated research organization (IRO) is an emerging model that is allowing multi-specialty practices and community health systems to be involved in clinical trials as a research care option for their patients. Privia Health, Tryon Medical Partners in Charlotte, North Carolina, and the AMC of Wake Forest University Health, for example, are benefitting through partnership with IRO Javara Research to participate in COVID-19 vaccine trials which other health systems in the same geographic areas in some cases have not managed to secure. We can expect to see these integrated research partnerships become more common as other health systems and medical practices recognize the high-profile COVID-19 studies that IRO partners have been able to win.
In the race against time, during a pandemic that has, to date, claimed nearly 250,000 lives, sponsors and CROs are placing their COVID-19 clinical trials in the same places that they have been doing so for decades. Site networks and academic medical centers continue to dominate- and the majority are based in the US. Independent medical practices, health systems, and government entities are playing a relatively small role in late stage COVID-19 clinical trials. Sponsor company site placement practices may likely contribute to participant disparities by race and ethnicity typically seen in pre-pandemic clinical trials unless remedial and innovative approaches are utilized.
References
- https://www.appliedclinicaltrialsonline.com/view/principal-investigators-take-lead-fda-regulated-studies
- Getz KA. Industry Trials Poised to Win Back Academia. Applied Clinical Trials. 2007; 0(0) para. 3, 22. Retrieved October 8, 2020, from https://www.appliedclinicaltrialsonline.com/view/industry-trials-poised-win-back-academia
- Getz KA, Lamberti MJ, Chakravarthy R. Assessing Practices & Inefficiencies with Site Selection, Study Start-Up, and Site Activation. Applied Clinical Trials. 2016; para. 4. Retrieved October 8, 2020, from https://www.appliedclinicaltrialsonline.com/view/limited-boosts-study-start
- Salzman S. The VA Gets Real on Reform. Applied Clinical Trials. 2019; 28(9): para. 3. Retrieved October 30, 2020, from https://www.appliedclinicaltrialsonline.com/view/va-gets-real-reform
- Chastain DB, Osae SP, Henao-Martínez AF, Franco-Paredes C, Chastain JS, Young GN, et al. Racial Disproportionality in Covid Clinical Trials. The New England Journal of Medicine. 2020; 383(9) e59-e59. Retrieved October 30, 2020, from https://www.nejm.org/doi/full/10.1056/NEJMp2021971
- Paulsen E, Practice-Based Clinical Trials: Benefits and Barriers. Duke Health. 2016. Retrieved October 30, 2020 from https://physicians.dukehealth.org/articles/practice-based-clinical-trials-benefits-and-barriers
- Kaplan GA, Weaver R, Cloud B. Clinical Trials: Insights from the Inside. Applied Clinical Trials. 2020; para. 13. Retrieved October 30, 2020, from https://www.appliedclinicaltrialsonline.com/view/effect-covid-19-clinical-trials-insights-inside
- Glabman, M. Community hospitals branch out to clinical research. ACP Hospitalist. 2008; para. 8. Retrieved October 30, 2020, from https://acphospitalist.org/archives/2008/12/research.htm
Noah Gottlieb, Jennifer Byrne; both with Javara Research, Kenneth Getz, Tufts Center for the Study of Drug Development
Originally published on: Applied Clinical Trials
